Imagine when someone throws a stone to you. It feels painful isn't it. Imagine all of your classmates throw stones at you, will you feel less or more painful? That's the same thing with gas. Gas molecules moving at a high speed in a large number will cause greater pressure on a wall or surface (but considerably less than that of the stones perhaps??)
2. Kinetic Theory of gases
The kinetic theory of gases is based on the following assumptions:
The molecules in a gas move freely in random motions and possess kinetic energy.
The forces of attraction between the molecules are negligible
The collisions of the molecules with each other and with the walls of the container are of elastic collisions.
The molecules of a gas in a container move in all directions to fill the entire space of the container
until they collide with its walls.
The collisions of the gas molecules with the walls of the container are elastic collisions and the molecules rebound with the same speed which results in a change in momentum for each molecule.
The total change of momentum when the gas molecules collide with the walls of the container in one second produces a force which acts on the walls of the container.
By the definition of Pressure = Force / Area (P = F/A)
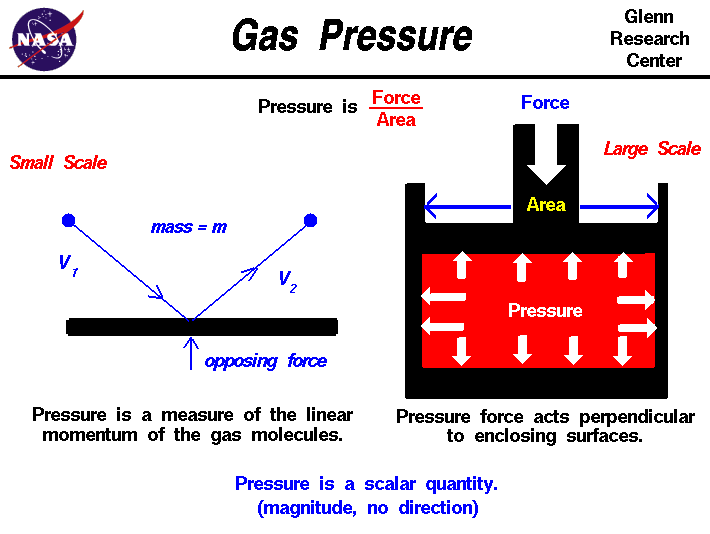
3. Factors Affecting Air or Gas Pressure
a. Pressure increases when the density of gas increases.
b. Pressure increases when temperature increases due to kinetic energy of molecules increases.
Atmospheric Pressure
1. Existence of Atmospheric pressure
.According to the kinetic theory of gases, gases consist of molecules which are far apart and in random motion at high speeds.
The gas molecules have mass and experience the gravitational pull. The result is that gases
have weight.
The atmosphere is a thick layer of air that surrounds the Earth. You may probably have known this.
The atmosphere exerts a pressure called atmospheric pressure which is caused by the weight of the thick layer of air above the Earth's surface.
Atmospheric pressure acts on every object on the surface of the earth. No one is in exception.
Activity to show the existence of Atmospheric Pressure
Fill a glass with water to the brim. Cover it with a thick cardboard. Invert it downwards. The water does not fall down. Why? because atmospheric pressure supports the cardboard (and water) from falling. The resultant force on the cardboard is greater than the weight of water. Even in the existence of gravity!
Boil an empty tin half-filled with water. Cap the tin. Let it cool under running tap water. Wallaa....the tin will get crumpled as the water cools down. As the steam condenses, the pressure
inside the metal tin decreases, The external atmospheric pressure which is Higher, crushes the tin.
Mercury Barometer
1. A mercury barometer consists of a thick-walled glass tube, which is closed at one end.
2. The tube is completely filled with mercury and inverted several times to remove air bubbles.
The tube is then completely filled again with mercury.
3. After all air has been removed, the open end of the glass tube is inverted into a container of mercury.
4. The mercury column drops until it reaches a height of about 76cm above the lower surface. The space between the top of the mercury and the end of the tube should contain no air; it must be in a complete vacuum.
5. The column of mercury in the tube is supported by the atmospheric pressure and its height depends on the magnitude of the atmospheric pressure.
6. Since the atmospheric pressure at sea level can support a vertical column of mercury 76 cm or 760 mm high, we can, for convenience, express mm Hg as a unit of pressure. 1 Standard atmospheric pressure (1 P atm) = 76 cm Hg or 760 mm Hg (also known as one atmosphere).
P atm = 76 cm Hg = 10 000 Pa.
1 P atm = 76 cm Hg = 10 000 Pa = 1 bar.
7. In unit m water: P atm = 10 m water.
7 comments:
Nice ex.amples
learnt so much thanks
How do you have gas pressure; without a container? Because...
'Gravity' is pseudoscience. Even Newton himself said: "I feign no hypothesis." Therefore, putting it in the pseudoscience category. So "MaSs AtTrAcKtInG MaSs." Isn't verified.
No valid independent variable. So, without that... "pulling" - how do you have gas PRESSURE without a container? Otherwise - NASA debunked themselves. You can't have gas pressure, beside a vacuum - without a container.
Thank you.
Gas will ALWAYS FILL the available space. If not... it's a 2nd law of thermodynamic violation.
So: get some truth in you. Here's something to get you started! Have a nice day.
https://youtu.be/aQ7_9eIAlT4
Thermodynamics******
earth is flat
Please stop with the "Quantum Erasaer" nonsense.
People come here to looking to learn actual science.
Post a Comment